الفرق بين المراجعتين لصفحة: «كودئين»
(قالب دواء، قالب أدوية سعال قالب أدوية إسهال، <!-- تضمين نص إنكليزي -->) |
(روابط) |
||
| سطر 61: | سطر 61: | ||
{{TOC limit|3}} | {{TOC limit|3}} | ||
== | ==الاستعمالات الطبية== | ||
Codeine is used to treat mild to moderate [[pain]] and to relieve [[cough]].<ref name=AHFS>{{cite web|title=Codeine|url=http://www.drugs.com/monograph/codeine.html|work=The American Society of Health-System Pharmacists|accessdate=3 April 2011}}</ref> Codeine is also used to treat [[diarrhea]] and diarrhea predominant [[irritable bowel syndrome]], although [[loperamide]] (which is available [[Over-the-counter drug|OTC]] for milder diarrhea), [[diphenoxylate]], [[paregoric]] or even [[laudanum]] (also known as ''Tincture of Opium'') are more frequently used to treat severe diarrhea.<ref>{{cite book |title=Diarrhea: Diagnostic and Therapeutic Advances|publisher=Humana Press |date= November 8, 2010 | location = New York, USA|isbn=1-60761-182-1|page=452 |url=http://books.google.com/?id=BstHGdtpb9AC&printsec=frontcover&dq=Diarrhea:+Diagnostic+and+Therapeutic+Advances#v=onepage&q&f=false|author=Stefano Guandalini; Haleh Vaziri}}</ref> | Codeine is used to treat mild to moderate [[pain]] and to relieve [[cough]].<ref name=AHFS>{{cite web|title=Codeine|url=http://www.drugs.com/monograph/codeine.html|work=The American Society of Health-System Pharmacists|accessdate=3 April 2011}}</ref> Codeine is also used to treat [[diarrhea]] and diarrhea predominant [[irritable bowel syndrome]], although [[loperamide]] (which is available [[Over-the-counter drug|OTC]] for milder diarrhea), [[diphenoxylate]], [[paregoric]] or even [[laudanum]] (also known as ''Tincture of Opium'') are more frequently used to treat severe diarrhea.<ref>{{cite book |title=Diarrhea: Diagnostic and Therapeutic Advances|publisher=Humana Press |date= November 8, 2010 | location = New York, USA|isbn=1-60761-182-1|page=452 |url=http://books.google.com/?id=BstHGdtpb9AC&printsec=frontcover&dq=Diarrhea:+Diagnostic+and+Therapeutic+Advances#v=onepage&q&f=false|author=Stefano Guandalini; Haleh Vaziri}}</ref> | ||
=== | ===المستحضرات=== | ||
Codeine is marketed as both a single-ingredient drug and in combination preparations with [[paracetamol]] (as [[co-codamol]]: ''e.g.,'' brands Paracod, Panadeine. Paramol, and the Tylenol-with-codeine series, including [[Tylenol 3]] and 1,2,4); with [[aspirin]]; (as [[co-codaprin]]); or with [[ibuprofen]] (as [[Nurofen Plus]]). These combinations provide greater pain relief than either agent alone (drug [[synergy]]). Codeine is also commonly marketed in products containing codeine with other pain killers or muscle relaxers, as well as codeine mixed with [[phenacetin]] (Emprazil With Codeine No. 1, 2, 3, and 4), [[naproxen]], [[indomethacin]], [[diclofenac]], and others, as well as more complex mixtures, including such mixtures as aspirin + paracetamol + codeine ± caffeine ± antihistamines and other agents, such as those mentioned above. | Codeine is marketed as both a single-ingredient drug and in combination preparations with [[paracetamol]] (as [[co-codamol]]: ''e.g.,'' brands Paracod, Panadeine. Paramol, and the Tylenol-with-codeine series, including [[Tylenol 3]] and 1,2,4); with [[aspirin]]; (as [[co-codaprin]]); or with [[ibuprofen]] (as [[Nurofen Plus]]). These combinations provide greater pain relief than either agent alone (drug [[synergy]]). Codeine is also commonly marketed in products containing codeine with other pain killers or muscle relaxers, as well as codeine mixed with [[phenacetin]] (Emprazil With Codeine No. 1, 2, 3, and 4), [[naproxen]], [[indomethacin]], [[diclofenac]], and others, as well as more complex mixtures, including such mixtures as aspirin + paracetamol + codeine ± caffeine ± antihistamines and other agents, such as those mentioned above. | ||
| سطر 71: | سطر 71: | ||
Injectable codeine is available for subcutaneous or intramuscular injection; intravenous injection can cause a serious reaction that can progress to [[anaphylaxis]]. Codeine suppositories are also marketed in some countries. | Injectable codeine is available for subcutaneous or intramuscular injection; intravenous injection can cause a serious reaction that can progress to [[anaphylaxis]]. Codeine suppositories are also marketed in some countries. | ||
== | ==الآثار الضارة== | ||
Common adverse effects associated with the use of codeine include [[drowsiness]] and [[constipation]]. Less common are [[Euphoria (emotion)|euphoria]], [[itching]], [[nausea]], [[vomiting]], [[dry mouth]], [[miosis]], [[orthostatic hypotension]], [[urinary retention]], [[Depression (mood)|depression]], and, paradoxically, [[coughing]]. Rare adverse effects include [[anaphylaxis]], [[Epileptic seizure|seizure]], and [[Hypoventilation|respiratory depression]].<ref>{{Cite web|publisher=WebMD LLC.|title=Codeine - adverse effects|url=http://reference.medscape.com/drug/codeine-343310#4|work=Medscape reference - Drugs, Diseases & Procedures|accessdate=27 Sep 2012}}</ref> As with all opiates, longer-term effects can vary but can include diminished libido, apathy and memory loss. Some people may also have an allergic reaction to codeine, such as the swelling of skin and rashes.<ref name=Drugs.com>[http://www.drugs.com/codeine.html Codeine Information from Drugs.com]</ref> | Common adverse effects associated with the use of codeine include [[drowsiness]] and [[constipation]]. Less common are [[Euphoria (emotion)|euphoria]], [[itching]], [[nausea]], [[vomiting]], [[dry mouth]], [[miosis]], [[orthostatic hypotension]], [[urinary retention]], [[Depression (mood)|depression]], and, paradoxically, [[coughing]]. Rare adverse effects include [[anaphylaxis]], [[Epileptic seizure|seizure]], and [[Hypoventilation|respiratory depression]].<ref>{{Cite web|publisher=WebMD LLC.|title=Codeine - adverse effects|url=http://reference.medscape.com/drug/codeine-343310#4|work=Medscape reference - Drugs, Diseases & Procedures|accessdate=27 Sep 2012}}</ref> As with all opiates, longer-term effects can vary but can include diminished libido, apathy and memory loss. Some people may also have an allergic reaction to codeine, such as the swelling of skin and rashes.<ref name=Drugs.com>[http://www.drugs.com/codeine.html Codeine Information from Drugs.com]</ref> | ||
| سطر 81: | سطر 81: | ||
In August 2012, the Federal Drug Administration issued a warning about deaths in pediatric patients < 6 years old after ingesting "normal" doses of acetaminophen with codeine after tonsillectomy.<ref>{{cite web | url = http://www.fda.gov/Drugs/DrugSafety/ucm313631.htm#professionals | title = FDA Drug Safety Communication: Codeine use in certain children after tonsillectomy and/or adenoidectomy may lead to rare, but life-threatening adverse events or death | publisher = [[Food and Drug Administration]]}}</ref> Some patients are very effective converters of codeine to its active form, hydromorphone, resulting in lethal blood levels. The FDA presently is recommending very cautious use of Codeine in young tonsillectomy patients: use the drug in the lowest amount that can control the pain, use "as needed" and not "around the clock", and seek immediate medical attention if a child on codeine exhibits excessive sedation or abnormally noisy breathing. | In August 2012, the Federal Drug Administration issued a warning about deaths in pediatric patients < 6 years old after ingesting "normal" doses of acetaminophen with codeine after tonsillectomy.<ref>{{cite web | url = http://www.fda.gov/Drugs/DrugSafety/ucm313631.htm#professionals | title = FDA Drug Safety Communication: Codeine use in certain children after tonsillectomy and/or adenoidectomy may lead to rare, but life-threatening adverse events or death | publisher = [[Food and Drug Administration]]}}</ref> Some patients are very effective converters of codeine to its active form, hydromorphone, resulting in lethal blood levels. The FDA presently is recommending very cautious use of Codeine in young tonsillectomy patients: use the drug in the lowest amount that can control the pain, use "as needed" and not "around the clock", and seek immediate medical attention if a child on codeine exhibits excessive sedation or abnormally noisy breathing. | ||
=== | ===الانسحاب والاعتماد=== | ||
As with other opiate-based pain killers, chronic use of codeine can cause [[physical dependence]]. When physical dependence has developed, withdrawal symptoms may occur if a person suddenly stops the medication. Withdrawal symptoms include: drug craving, runny nose, yawning, sweating, insomnia, weakness, stomach cramps, nausea, vomiting, diarrhea, muscle spasms, chills, irritability, and pain. To minimize withdrawal symptoms, long-term users should gradually reduce their codeine medication under the supervision of a healthcare professional.<ref>{{cite web| url = http://www.aadac.com/87_436.asp | title = The ABCs - Codeine and Other Opioid Painkillers | accessdate = Sep 12 2008 | author = Alberta Health Services | authorlink = Alberta Health Services | coauthors = AADAC | date = April 16, 2007 | publisher = Alberta Alcohol and Drug Abuse Commission }}</ref> | As with other opiate-based pain killers, chronic use of codeine can cause [[physical dependence]]. When physical dependence has developed, withdrawal symptoms may occur if a person suddenly stops the medication. Withdrawal symptoms include: drug craving, runny nose, yawning, sweating, insomnia, weakness, stomach cramps, nausea, vomiting, diarrhea, muscle spasms, chills, irritability, and pain. To minimize withdrawal symptoms, long-term users should gradually reduce their codeine medication under the supervision of a healthcare professional.<ref>{{cite web| url = http://www.aadac.com/87_436.asp | title = The ABCs - Codeine and Other Opioid Painkillers | accessdate = Sep 12 2008 | author = Alberta Health Services | authorlink = Alberta Health Services | coauthors = AADAC | date = April 16, 2007 | publisher = Alberta Alcohol and Drug Abuse Commission }}</ref> | ||
Codeine is metabolized to [[codeine-6-glucuronide]] (C6G) by uridine diphosphate glucuronosyl transferase [[UGT2B7]], and, since only about 5% of codeine is metabolized by cytochrome P450 [[CYP2D6]], the current evidence is that C6G is the primary active compound.<ref>{{Cite journal|author=Armstrong SC, Cozza KL |title=Pharmacokinetic drug interactions of morphine, codeine, and their derivatives: theory and clinical reality, Part II |journal=Psychosomatics |volume=44 |issue=6 |pages=515–20 |year=2003 |pmid=14597688 |doi=10.1176/appi.psy.44.6.515}}</ref> Claims about the supposed "ceiling effect" of codeine doses are based on the assumption that high doses of codeine saturate CYP2D6, preventing further conversion of codeine to morphine, however it is now known that C6G is the main metabolite responsible for codeine's analgesia.<ref name="Vree2000">{{Cite journal|author=Vree TB, van Dongen RT, Koopman-Kimenai PM |title=Codeine analgesia is due to codeine-6-glucuronide, not morphine |journal=Int. J. Clin. Practa. |volume=54 |issue=6 |pages=395–8 |year=2000 |pmid=11092114 |doi=}}</ref> There is also no evidence that CYP2D6 inhibition is useful in treating codeine dependence,<ref>{{Cite journal|author=Fernandes LC, Kilicarslan T, Kaplan HL, Tyndale RF, Sellers EM, Romach MK |title=Treatment of codeine dependence with inhibitors of cytochrome P450 2D6 |journal=J Clin Psychopharmacol |volume=22 |issue=3 |pages=326–9 |year=2002 |month=June |pmid=12006904 |doi=10.1097/00004714-200206000-00014}}</ref> though the metabolism of codeine to morphine (and hence further metabolism to glucuronide morphine conjugates) does have an effect on the abuse potential of codeine.<ref>{{Cite journal|doi=10.1097/00004714-200008000-00008 |author=Kathiramalainathan K, Kaplan HL, Romach MK, ''et al.'' |title=Inhibition of cytochrome P450 2D6 modifies codeine abuse liability |journal=J Clin Psychopharmacol |volume=20 |issue=4 |pages=435–44 |year=2000 |pmid=10917405}}</ref> However, CYP2D6 has been implicated in the toxicity and death of neonates when codeine is administered to lactating mothers, particularly those with increased 2D6 activity ("ultra-rapid" metabolizers).<ref>{{Cite journal|pmid=16920476|author=Koren G, Cairns J, Chitayat D, Gaedigk A, Leeder SJ |title=Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine-prescribed mother|journal=Lancet |volume=368 |issue=9536 |page=704 |year=2006 |doi=10.1016/S0140-6736(06)69255-6}}</ref><ref>{{Cite journal|doi=10.1038/clpt.2009.151 |author=Willmann S, Edginton AN, Coboeken K, Ahr G, Lippert J |title=Risk to the Breast-Fed Neonate From Codeine Treatment to the Mother: A Quantitative Mechanistic Modeling Study|journal=Clinical Pharmacology & Therapeutics |volume=86 |issue=6 |pages=634–43 |year=2009}}</ref> | Codeine is metabolized to [[codeine-6-glucuronide]] (C6G) by uridine diphosphate glucuronosyl transferase [[UGT2B7]], and, since only about 5% of codeine is metabolized by cytochrome P450 [[CYP2D6]], the current evidence is that C6G is the primary active compound.<ref>{{Cite journal|author=Armstrong SC, Cozza KL |title=Pharmacokinetic drug interactions of morphine, codeine, and their derivatives: theory and clinical reality, Part II |journal=Psychosomatics |volume=44 |issue=6 |pages=515–20 |year=2003 |pmid=14597688 |doi=10.1176/appi.psy.44.6.515}}</ref> Claims about the supposed "ceiling effect" of codeine doses are based on the assumption that high doses of codeine saturate CYP2D6, preventing further conversion of codeine to morphine, however it is now known that C6G is the main metabolite responsible for codeine's analgesia.<ref name="Vree2000">{{Cite journal|author=Vree TB, van Dongen RT, Koopman-Kimenai PM |title=Codeine analgesia is due to codeine-6-glucuronide, not morphine |journal=Int. J. Clin. Practa. |volume=54 |issue=6 |pages=395–8 |year=2000 |pmid=11092114 |doi=}}</ref> There is also no evidence that CYP2D6 inhibition is useful in treating codeine dependence,<ref>{{Cite journal|author=Fernandes LC, Kilicarslan T, Kaplan HL, Tyndale RF, Sellers EM, Romach MK |title=Treatment of codeine dependence with inhibitors of cytochrome P450 2D6 |journal=J Clin Psychopharmacol |volume=22 |issue=3 |pages=326–9 |year=2002 |month=June |pmid=12006904 |doi=10.1097/00004714-200206000-00014}}</ref> though the metabolism of codeine to morphine (and hence further metabolism to glucuronide morphine conjugates) does have an effect on the abuse potential of codeine.<ref>{{Cite journal|doi=10.1097/00004714-200008000-00008 |author=Kathiramalainathan K, Kaplan HL, Romach MK, ''et al.'' |title=Inhibition of cytochrome P450 2D6 modifies codeine abuse liability |journal=J Clin Psychopharmacol |volume=20 |issue=4 |pages=435–44 |year=2000 |pmid=10917405}}</ref> However, CYP2D6 has been implicated in the toxicity and death of neonates when codeine is administered to lactating mothers, particularly those with increased 2D6 activity ("ultra-rapid" metabolizers).<ref>{{Cite journal|pmid=16920476|author=Koren G, Cairns J, Chitayat D, Gaedigk A, Leeder SJ |title=Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine-prescribed mother|journal=Lancet |volume=368 |issue=9536 |page=704 |year=2006 |doi=10.1016/S0140-6736(06)69255-6}}</ref><ref>{{Cite journal|doi=10.1038/clpt.2009.151 |author=Willmann S, Edginton AN, Coboeken K, Ahr G, Lippert J |title=Risk to the Breast-Fed Neonate From Codeine Treatment to the Mother: A Quantitative Mechanistic Modeling Study|journal=Clinical Pharmacology & Therapeutics |volume=86 |issue=6 |pages=634–43 |year=2009}}</ref> | ||
==Pharmacokinetics== | ==الحرائك الدوائية Pharmacokinetics== | ||
The conversion of codeine to morphine occurs in the liver and is catalysed by the [[cytochrome P450]] enzyme [[CYP2D6]]. [[CYP3A4]] produces norcodeine and [[UGT2B7]] conjugates codeine, norcodeine, and morphine to the corresponding 3- and 6- glucuronides. Approximately 6–10% of the Caucasians, 2% of Asians, and 1% of Arabs<ref>{{cite web|url=http://codeine.50g.com/info/codeine.html |title=Codeine Information - Facts - Codeine |accessdate=2007-07-16 |work=}}</ref> are "poor metabolizers"; they have little CYP2D6, and codeine is less effective for analgesia in these patients (Rossi, 2004). Srinivasan, Wielbo and Tebbett speculate that codeine-6-glucuronide is responsible for a large percentage of the analgesia of codeine, and, thus, these patients should experience some analgesia.<ref name="Srinivasan V, Wielbo D, Tebbett IR 1997 185–90">{{Cite journal|author=Srinivasan V, Wielbo D, Tebbett IR |title=Analgesic effects of codeine-6-glucuronide after intravenous administration |journal=European Journal of Pain |volume=1 |issue=3 |pages=185–90 |year=1997 |pmid=15102399 |doi=10.1016/S1090-3801(97)90103-8}}</ref> Many of the adverse effects will still be experienced in poor metabolizers. Conversely, 0.5-2% of the population are "extensive metabolizers"; multiple copies of the gene for 2D6 produce high levels of CYP2D6 and will metabolize drugs through that pathway more quickly than others. | The conversion of codeine to morphine occurs in the liver and is catalysed by the [[cytochrome P450]] enzyme [[CYP2D6]]. [[CYP3A4]] produces norcodeine and [[UGT2B7]] conjugates codeine, norcodeine, and morphine to the corresponding 3- and 6- glucuronides. Approximately 6–10% of the Caucasians, 2% of Asians, and 1% of Arabs<ref>{{cite web|url=http://codeine.50g.com/info/codeine.html |title=Codeine Information - Facts - Codeine |accessdate=2007-07-16 |work=}}</ref> are "poor metabolizers"; they have little CYP2D6, and codeine is less effective for analgesia in these patients (Rossi, 2004). Srinivasan, Wielbo and Tebbett speculate that codeine-6-glucuronide is responsible for a large percentage of the analgesia of codeine, and, thus, these patients should experience some analgesia.<ref name="Srinivasan V, Wielbo D, Tebbett IR 1997 185–90">{{Cite journal|author=Srinivasan V, Wielbo D, Tebbett IR |title=Analgesic effects of codeine-6-glucuronide after intravenous administration |journal=European Journal of Pain |volume=1 |issue=3 |pages=185–90 |year=1997 |pmid=15102399 |doi=10.1016/S1090-3801(97)90103-8}}</ref> Many of the adverse effects will still be experienced in poor metabolizers. Conversely, 0.5-2% of the population are "extensive metabolizers"; multiple copies of the gene for 2D6 produce high levels of CYP2D6 and will metabolize drugs through that pathway more quickly than others. | ||
| سطر 95: | سطر 95: | ||
The active metabolites of codeine, notably morphine, exert their effects by binding to and activating the μ-[[opioid receptor]]. | The active metabolites of codeine, notably morphine, exert their effects by binding to and activating the μ-[[opioid receptor]]. | ||
== | ==صلته بالأفيونات الأخرى== | ||
Codeine is the starting material and prototype of a large class of mainly mild to moderately strong opioids such as [[hydrocodone]] (1920 in Germany), [[oxycodone]] (1916 in Germany), [[dihydrocodeine]] (1908 in Germany), and its derivatives such as [[nicocodeine]] (1956 in Austria). Other series of codeine derivatives include isocodeine and its derivatives, which were developed in Germany starting around 1920. As an analgesic, codeine [[Opiate comparison|compares moderately to other opiates]]. Related to codeine in other ways are [[codoxime]], [[thebacon]], [[Codeine-N-oxide|codeine-''N''-oxide]] (genocodeine), related to the nitrogen morphine derivatives as is codeine methobromide, and [[heterocodeine]], which is a drug six times stronger than morphine and 72 times stronger than codeine due to a small re-arrangement of the molecule, viz. moving the methyl group from the 3 to the 6 position on the morphine carbon skeleton. Drugs bearing resemblance to codeine in effects due to close structural relationship are variations on the methyl groups at the 3 position including [[ethylmorphine]] a.k.a. codethyline (Dionine) and [[benzylmorphine]] (Peronine). While having no narcotic effects of its own, the important opioid precursor [[thebaine]] differs from codeine only slightly in structure. Pseudocodeine and some other similar alkaloids not currently used in medicine are found in trace amounts in opium as well. | Codeine is the starting material and prototype of a large class of mainly mild to moderately strong opioids such as [[hydrocodone]] (1920 in Germany), [[oxycodone]] (1916 in Germany), [[dihydrocodeine]] (1908 in Germany), and its derivatives such as [[nicocodeine]] (1956 in Austria). Other series of codeine derivatives include isocodeine and its derivatives, which were developed in Germany starting around 1920. As an analgesic, codeine [[Opiate comparison|compares moderately to other opiates]]. Related to codeine in other ways are [[codoxime]], [[thebacon]], [[Codeine-N-oxide|codeine-''N''-oxide]] (genocodeine), related to the nitrogen morphine derivatives as is codeine methobromide, and [[heterocodeine]], which is a drug six times stronger than morphine and 72 times stronger than codeine due to a small re-arrangement of the molecule, viz. moving the methyl group from the 3 to the 6 position on the morphine carbon skeleton. Drugs bearing resemblance to codeine in effects due to close structural relationship are variations on the methyl groups at the 3 position including [[ethylmorphine]] a.k.a. codethyline (Dionine) and [[benzylmorphine]] (Peronine). While having no narcotic effects of its own, the important opioid precursor [[thebaine]] differs from codeine only slightly in structure. Pseudocodeine and some other similar alkaloids not currently used in medicine are found in trace amounts in opium as well. | ||
== | ==تاريخه== | ||
Codeine, or 3-methylmorphine, is an [[alkaloid]] found in the [[opium poppy]], ''Papaver somniferum var. album'', a plant in the [[papaveraceae]] family. Opium poppy has been cultivated and utilized throughout human history for a variety of medicinal (analgesic, anti-tussive and anti-diarrheal) and hypnotic properties linked to the diversity of its active components, which include morphine, codeine and [[papaverine]]. | Codeine, or 3-methylmorphine, is an [[alkaloid]] found in the [[opium poppy]], ''Papaver somniferum var. album'', a plant in the [[papaveraceae]] family. Opium poppy has been cultivated and utilized throughout human history for a variety of medicinal (analgesic, anti-tussive and anti-diarrheal) and hypnotic properties linked to the diversity of its active components, which include morphine, codeine and [[papaverine]]. | ||
| سطر 126: | سطر 126: | ||
Codeine is also demethylated by reaction with [[pyridine]] to illicitly synthesize morphine, which can then be acetylated to make heroin (diacetylmorphine). Pyridine is toxic and possibly [[carcinogenic]], so morphine illicitly produced in this manner (and potentially contaminated with pyridine) may be particularly harmful.<ref>{{Cite book| last = Hogshire | first = Jim | authorlink = Jim Hogshire | title = Pills-A-Go-Go: A Fiendish Investigation into Pill Marketing, Art, History & Consumption | publisher = Feral House | year = 1999 | month = June | location = Los Angeles | pages = 216–223 | isbn = 978-0-922915-53-8}}</ref> Codeine can also be turned into [[α-chlorocodide]], which is used in the clandestine synthesis of [[desomorphine]] (Permonid) (desomorphine attracted attention in 2010 in [[Russia]] due to an upsurge in clandestine production, presumably due to its relatively simple synthesis from codeine.{{Citation needed|date=November 2011}} The drug is easily made from codeine, [[iodine]] and [[red phosphorus]],<ref>{{cite journal |pages=361–70 |doi=10.1134/S1061934808040096 |title=Chromatographic study of expert and biological samples containing desomorphine |year=2011 |last1=Savchuk |first1=S. A. |last2=Barsegyan |first2=S. S. |last3=Barsegyan |first3=I. B. |last4=Kolesov |first4=G. M. |journal=Journal of Analytical Chemistry |volume=63 |issue=4}}</ref> in a similar process to the manufacture of [[methamphetamine]] from [[pseudoephedrine]], but desomorphine made this way is highly impure and contaminated with various toxic and corrosive byproducts.). | Codeine is also demethylated by reaction with [[pyridine]] to illicitly synthesize morphine, which can then be acetylated to make heroin (diacetylmorphine). Pyridine is toxic and possibly [[carcinogenic]], so morphine illicitly produced in this manner (and potentially contaminated with pyridine) may be particularly harmful.<ref>{{Cite book| last = Hogshire | first = Jim | authorlink = Jim Hogshire | title = Pills-A-Go-Go: A Fiendish Investigation into Pill Marketing, Art, History & Consumption | publisher = Feral House | year = 1999 | month = June | location = Los Angeles | pages = 216–223 | isbn = 978-0-922915-53-8}}</ref> Codeine can also be turned into [[α-chlorocodide]], which is used in the clandestine synthesis of [[desomorphine]] (Permonid) (desomorphine attracted attention in 2010 in [[Russia]] due to an upsurge in clandestine production, presumably due to its relatively simple synthesis from codeine.{{Citation needed|date=November 2011}} The drug is easily made from codeine, [[iodine]] and [[red phosphorus]],<ref>{{cite journal |pages=361–70 |doi=10.1134/S1061934808040096 |title=Chromatographic study of expert and biological samples containing desomorphine |year=2011 |last1=Savchuk |first1=S. A. |last2=Barsegyan |first2=S. S. |last3=Barsegyan |first3=I. B. |last4=Kolesov |first4=G. M. |journal=Journal of Analytical Chemistry |volume=63 |issue=4}}</ref> in a similar process to the manufacture of [[methamphetamine]] from [[pseudoephedrine]], but desomorphine made this way is highly impure and contaminated with various toxic and corrosive byproducts.). | ||
=== | ===التحري=== | ||
Codeine and/or its major metabolites may be quantitated in blood, plasma or urine to monitor therapy, confirm a diagnosis of poisoning or assist in a medicolegal death investigation. Drug abuse screening programs generally test urine, hair, sweat or oral fluid. Many commercial opiate screening tests directed at morphine cross-react appreciably with codeine and its metabolites, but chromatographic techniques can easily distinguish codeine from other opiates and opioids. It is important to note that codeine usage results in significant amounts of morphine as an excretion product. Furthermore, heroin contains codeine (or acetylcodeine) as an impurity and its use will result in excretion of small amounts of codeine. Poppy seed foods represent yet another source of low levels of codeine in one's biofluids. Blood or plasma codeine concentrations are typically in the 50–300 µg/L range in persons taking the drug therapeutically, 700–7000 µg/L in chronic users and 1000–10,000 µg/L in cases of acute fatal overdosage.<ref>{{cite journal |author=Thevis M, Opfermann G, Schänzer W |title=Urinary concentrations of morphine and codeine after consumption of poppy seeds |journal=J. Anal. Toxicol. |volume=27 |pages=53–6 |year=2003 |pmid=12587685 |issue=1}}</ref><ref>{{cite journal |author=Cone EJ, Welch P, Paul BD, Mitchell JM |title=Forensic drug testing for opiates, III. Urinary excretion rates of morphine and codeine following codeine administration |journal=J. Anal. Toxicol. |volume=15 |pages=161–6 |year=1991 |pmid=1943064 |issue=4}}</ref><ref>{{cite book |author=Baselt, R. |title=Disposition of Toxic Drugs and Chemicals in Man |publisher=Biomedical Publications |location=Foster City CA |year=2008 |pages=355–360 |edition=8th }}</ref> | Codeine and/or its major metabolites may be quantitated in blood, plasma or urine to monitor therapy, confirm a diagnosis of poisoning or assist in a medicolegal death investigation. Drug abuse screening programs generally test urine, hair, sweat or oral fluid. Many commercial opiate screening tests directed at morphine cross-react appreciably with codeine and its metabolites, but chromatographic techniques can easily distinguish codeine from other opiates and opioids. It is important to note that codeine usage results in significant amounts of morphine as an excretion product. Furthermore, heroin contains codeine (or acetylcodeine) as an impurity and its use will result in excretion of small amounts of codeine. Poppy seed foods represent yet another source of low levels of codeine in one's biofluids. Blood or plasma codeine concentrations are typically in the 50–300 µg/L range in persons taking the drug therapeutically, 700–7000 µg/L in chronic users and 1000–10,000 µg/L in cases of acute fatal overdosage.<ref>{{cite journal |author=Thevis M, Opfermann G, Schänzer W |title=Urinary concentrations of morphine and codeine after consumption of poppy seeds |journal=J. Anal. Toxicol. |volume=27 |pages=53–6 |year=2003 |pmid=12587685 |issue=1}}</ref><ref>{{cite journal |author=Cone EJ, Welch P, Paul BD, Mitchell JM |title=Forensic drug testing for opiates, III. Urinary excretion rates of morphine and codeine following codeine administration |journal=J. Anal. Toxicol. |volume=15 |pages=161–6 |year=1991 |pmid=1943064 |issue=4}}</ref><ref>{{cite book |author=Baselt, R. |title=Disposition of Toxic Drugs and Chemicals in Man |publisher=Biomedical Publications |location=Foster City CA |year=2008 |pages=355–360 |edition=8th }}</ref> | ||
== | ==الوضع القانوني== | ||
In [[Australia]], [[Canada]], [[New Zealand]], [[Romania]], [[Russia]], [[Sweden]], the [[United Kingdom]], the [[United States]], and many other countries, codeine is regulated under various [[Drug prohibition law#List by jurisdiction of principal drug prohibition laws|narcotic control laws]]. In some countries it is available without prescription in combination preparations from licensed pharmacists in doses up to 15 mg/tablet in Australia, New Zealand, Poland, Romania (Codamin), and Costa Rica, 12.8 mg/tablet in the UK, 10 mg/tablet in Israel and 8 mg/tablet in Canada and Estonia.{{Citation needed|date=June 2007}}<!--I did find this unsupported reference "it is available without prescription in combination preparations from licensed pharmacists in doses up to 8 mg/tablet in Canada and Australia, 15 mg/tablet in New Zealand and 12.8mg/tablet in Ireland and the UK (with paracetamol or ibuprofen), 9.6 mg/tablet in Denmark (with acetylosalicic acid and magnesium hydroxide making it unfit for cold water extraction). For Ireland, the ceiling is 20 mg per doseage unit. "-->. | In [[Australia]], [[Canada]], [[New Zealand]], [[Romania]], [[Russia]], [[Sweden]], the [[United Kingdom]], the [[United States]], and many other countries, codeine is regulated under various [[Drug prohibition law#List by jurisdiction of principal drug prohibition laws|narcotic control laws]]. In some countries it is available without prescription in combination preparations from licensed pharmacists in doses up to 15 mg/tablet in Australia, New Zealand, Poland, Romania (Codamin), and Costa Rica, 12.8 mg/tablet in the UK, 10 mg/tablet in Israel and 8 mg/tablet in Canada and Estonia.{{Citation needed|date=June 2007}}<!--I did find this unsupported reference "it is available without prescription in combination preparations from licensed pharmacists in doses up to 8 mg/tablet in Canada and Australia, 15 mg/tablet in New Zealand and 12.8mg/tablet in Ireland and the UK (with paracetamol or ibuprofen), 9.6 mg/tablet in Denmark (with acetylosalicic acid and magnesium hydroxide making it unfit for cold water extraction). For Ireland, the ceiling is 20 mg per doseage unit. "-->. | ||
<!-- | <!-- | ||
| سطر 205: | سطر 205: | ||
Under the Misues of Drugs Act 1971, possession of codeine is legal without a prescription in quantities of up to 12.5 mg when in tablet form. As with most opioids, possession of neat codeine without a prescripion is illegal in quantities over 12.5 mg and is currently a class B controlled drug. However, if it prepared for intra-muscular injection, it is then considered to be a class A controlled drug. Thus it is legal for a person to carry neat codeine in quantities over 12.5 mg assuming that they possess a valid prescription, subject to the quantities carried being for personal use only and with no indication that there is intent to supply.<ref>http://www.legislation.gov.uk/ukpga/1971/38/schedule/2</ref> | Under the Misues of Drugs Act 1971, possession of codeine is legal without a prescription in quantities of up to 12.5 mg when in tablet form. As with most opioids, possession of neat codeine without a prescripion is illegal in quantities over 12.5 mg and is currently a class B controlled drug. However, if it prepared for intra-muscular injection, it is then considered to be a class A controlled drug. Thus it is legal for a person to carry neat codeine in quantities over 12.5 mg assuming that they possess a valid prescription, subject to the quantities carried being for personal use only and with no indication that there is intent to supply.<ref>http://www.legislation.gov.uk/ukpga/1971/38/schedule/2</ref> | ||
--> | --> | ||
| سطر 309: | سطر 292: | ||
==مصادر== | ==مصادر== | ||
{{ثبت المراجع}} | {{ثبت المراجع}} | ||
كتاب الشامل في الأدوية السريرية، د.محمد عبد الرحمن العينية - د.محمود موسى طلوزي، منشورات دار القدس للعلوم | * كتاب الشامل في الأدوية السريرية، د.محمد عبد الرحمن العينية - د.محمود موسى طلوزي، منشورات دار القدس للعلوم | ||
* [http://en.wikipedia.org/wiki/Codeine مقالة كودئين - wikipedia] | |||
* [http://ar.wikipedia.org/wiki/%D9%83%D9%88%D8%AF%D9%8A%D9%86 مقالة كودئين - ويكيبيديا] | |||
{{بذرة}} | {{بذرة}} | ||
مراجعة 09:01، 26 أبريل 2013
| |
|---|---|
| Systematic (IUPAC) name | |
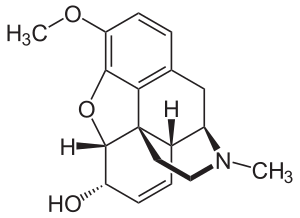
| (5α,6α)-7,8-didehydro-4,5-epoxy-3-methoxy-17-methylmorphinan-6-ol | |
| Clinical data | |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a682065 |
| Pregnancy cat. | C (US) |
| Legal status | Controlled (S8) (AU) Schedule I (CA) POM (UK) Schedule II (US) |
| Dependence liability | High |
| Routes | Oral, intra-rectally, SC, IM |
| Pharmacokinetic data | |
| Bioavailability | ~90% Oral |
| Metabolism | Hepatic, via CYP2D6 (cytochrome P450 2D6)[1] |
| Half-life | 2.5–3 h |
| Identifiers | |
| CAS number | 76-57-3 |
| ATC code | R05DA04 combinations: N02AA59, N02AA79 |
| PubChem | CID 5284371 |
| IUPHAR ligand | 1673 |
| DrugBank | DB00318 |
| ChemSpider | 4447447 |
| UNII | Q830PW7520 |
| KEGG | C06174 |
| ChEBI | CHEBI:16714 |
| ChEMBL | CHEMBL485 |
| Chemical data | |
| Formula | C18H21NO3 |
| Mol. mass | 299.364 g/mol |
| |
| |
| | |
.
الكودئين Codeine هو ميتيل مورفين، كودئين فوسفات، كودئين سلفات.
الزمرة الدوائية
مسكن أفيوني ومضاد سعال
الاستعمال
علاج الألم الخفيف إلى متوسط الشدة، وكمضاد سعال بجرعته المنخفضة.
الاستخدام خلال الحمل
ينتمي لأدوية المجموعة C، وينتمي بأدوية المجموعة D إذا استخدم لفترات طويلة أو بجرعات منخفضة.
مضادات الاستطباب
فرط الحساسية له أو لمكوناته.
تحذيرات
- استخدامه بحذر عند مرضى فرط الحساسية للشادات الأفيونية المشتقة من مجموعة فينانترين (مورفين هيدروكلوريد، هيدرومورفون، أوكسي كودون، أوكسي مورفون) أو المصابين بأحد الأمراض التنفسية كالربو أو النفاخ أو الداء الرئوي أو الساد المزمن أو المصابين بمرض شديد كبدي أو كلوي.
- قد تحوي بعض مستحضراته مادة السلفيت التي قد تسبب ارتكاست أرجية ملحوظة الشدة.
- لا ينصح باستخدامه لتثبيط السعال المنتج للقشع، ولا ينصح باستخدامه لتثبيط السعال عند الأطفال الذين تقل أعمارهم عن سنتين.
- قد يكون المريض المسن مؤهب بشكل كبير للإصابة بالتخليط والإمساك والتأثيرات المثبطة العصبية المركزية بقية التأثيرات الجانبية الناجمة عن الشادات الأفيونية.
الآثار الجانبية
- بنسبة تزيد عن 10%:
- قلبية وعائية: بطء/تسرع القلب، انخفاض التوتر الشرياني.
- عصبية مركزية: دوام، خفة الرأس، شعور كاذب بالتحسن، تعب، صداع، تململ تنبه عصبي مركزي تناقصي، تخليط.
- جلدية: شرى، طفح
- هضمية: جفاف الفم، قهم، غثيان، إقياء
- بولية تناسلية: نقص التبول، تشنج حالبي
- موضعية: حس حرق عند موضع الحقن
- عينية: تشوش الرؤية
- عصبية عضلية: ضعف عضلي
- تنفسية: زلة تنفسية
- متنوعة: تحرر الهيستامين
- بنسبة تقل عن 10%:
- عصبية مركزية: اختلاجات، إهلاس، اكتئاب، كوابيس، أرق
- هضمية: علوص شللي، تشنج صفراوي، معص معدي
فرط الجرعة/الانسمام
- تثبيط تنفسي وعصبي مركزي ومعص هضمي وإمساك.
- النالوكسون ترياق نوعي يستخدم للتدبير، يعطى حقناً وريدياً بجرعة 2ملغ (قد يستطب تكرارها عدة مرات) ، الجرعة القصوى 10ملغ.
التداخلات الدوائية
- تنخفض شدة التأثير عند المدخنين
- تزداد الفعالية والسمية عند إشراكه مع مثبطات الجملة العصبية والفينزتيازيدات أو TCAs أو أحد الشادات الأفيونية الأخرى أو غوانابنز أو مثبطات MAO أو المرخيات العضلية
الثبات
- يحفظ بدرجة 15-30م ولا يعرض للتجمد أو الضوء
- لا يستخدم محلوله المعد للحقن فيما و تغير لونه أو تحوصب
الآلية
-يرتبط مع المستقبلات الأفيونية في CNS مما يؤدي لحصار التوصيل عبر سبل الألم الصاعدة، كذلك فهو يؤثر على شدة الإحساس بالألم والارتكاس له. -يثبط السعال بالتأثير المباشر المركزي على البصلة السيسائية -ي4سبب تثبيط عصبي مركزي معمم.
الحرائك الدوائية
- بدء التأثير:
- فموي: 0.5-1 ساعة
- حقن عضلي: 10-30 دقيقة
- ذروة التأثير:
- فموي: 1-1.5 ساعة
- حقن عضلي: 0.5-1 ساعة
- مدة التأثير: 4-6 ساعات
- الامتصاص: كافي يلي تناوله عبر الفم
- التوزع: يعبر المشيمة، ويهر في حليب الأم
- الارتباط البروتيني: 7%
- الاستقلاب: يستقلب في الكبد متحولاً إلى مورفين
- العمر النصفي: 2.5-3.5 ساعة
- الإطراح: يطرح 3% إلى 16% مع البول على شكل دواء غير متبدل ونوركودئين ومورفين حر ومورفين مرتبط.
الجرعة
- يجب معايرة الجرعات حسب التأثير التسكيني المطلوب، إن نسبة فعالية الجرعة الفموية المسكنة تعادل ثلث فعالية نظيرتها الانحلالية.
- التسكين:
- الأطفال: يعطى فموي أو حقن عضلي تحت الجلد بجرعة 0.5-1 ملغ/كغ كل 4-6 ساعات حسب الحاجة، الجرعة القصوى 60ملغ كل 4-6 ساعات.
- البالغين: يعطى فموي أو حقن عضي تحت الجلد بجرعة 30ملغ (15-60 ملغ) كل 4-6 ساعات حسب الحاجة، الجرعة القصوى 360ملغ/24 ساعة.
- تثبيط السعال: فموي لتثبيط السعال غير المنتج للقشع
- الأطفال بجرعة 1 -1.5 ملغ/كغ/يوم تقسم 4-6 دفعات حسب الحاجة.
- البالغين: 10-20 ملغ كل 4-6 ساعات حسب الحاجة، الجرعة القصوى 120ملغ/24 ساعة.
تعدل الجرعة عند مريض القصور الكلوي (قد يعدل ذلك عند شدة المرض):
- تصفية الكرياتينين 10-50 مل/د: 75% من الجرعة المعتادة.
- تصفية الكرياتينين أقل من 10 مل: 50% من الجرعة المعتادة.
المراقبة
يراقب مدى زوال الألم، والحالة التنفسية والعقلية، والعلامات الحيوية.
ملاحظات
- يجب تجنب تناول مثبطات الجملة العصبية/الكحول خلال فترة الاستخدام بسبب تعاضد التأثير المثبط (تهدئة مفرطة)
- يراقب سكر الدم لأنه يسبب ارتفاع التركيز المصلي للسكر عند تناول الأطعمة الحاوية عليه
- قد يسبب النعاس وقد يلحق الخلل بإنجاز الأعمال التي تحتاج لتركيز ومحاكمة منطقية
- قد يسبب استعماله المديد اعتماد فيزيائي ونفسي ملحوظ
التداخلات المخبرية
يسبب تناوله ارتفاع التراكيز المصلية من SGOT وSGPT
الأشكال الصيدلانية
- محلول معد للحقن: 30ملغ (1مل، 2مل) – 60ملغ (1مل، 2مل)
- محلول معد للتناول عبر الفم: 15ملغ/5ملغ
- أقراص عادية: 15ملغ، 30ملغ، 60ملغ
- أقراص ذوابة: 15ملغ، 30ملغ، 60ملغ
مصادر
- كتاب الشامل في الأدوية السريرية، د.محمد عبد الرحمن العينية - د.محمود موسى طلوزي، منشورات دار القدس للعلوم
- مقالة كودئين - wikipedia
- مقالة كودئين - ويكيبيديا